Nye publikasjoner
Nytt apparat forbedrer generering av stamceller for behandling av Alzheimers sykdom
Sist anmeldt: 02.07.2025

Alt iLive-innhold blir gjennomgått med medisin eller faktisk kontrollert for å sikre så mye faktuell nøyaktighet som mulig.
Vi har strenge retningslinjer for innkjøp og kun kobling til anerkjente medieområder, akademiske forskningsinstitusjoner og, når det er mulig, medisinsk peer-evaluerte studier. Merk at tallene i parenteser ([1], [2], etc.) er klikkbare koblinger til disse studiene.
Hvis du føler at noe av innholdet vårt er unøyaktig, utdatert eller ellers tvilsomt, velg det og trykk Ctrl + Enter.

Forskere i Sverige sier de har perfeksjonert en teknikk for å konvertere vanlige hudceller til nevrale stamceller, noe de sier bringer dem nærmere rimelige, personlige celleterapier for Alzheimers og Parkinsonssykdom.
Ved hjelp av en spesialdesignet mikrofluidisk enhet har forskerteamet utviklet en enestående og akselerert tilnærming til å omprogrammere menneskelige hudceller til induserte pluripotente stamceller (iPSC-er) og deretter konvertere dem til nevrale stamceller.
Den første forfatteren av studien, Saumya Jain, sier at plattformen kan forbedre og redusere kostnadene for celleterapi ved å gjøre cellene mer kompatible og aksepterte av pasientens kropp. Studien ble publisert i tidsskriftet Advanced Science av forskere fra KTH Royal Institute of Technology.
Anna Herland, seniorforfatter av studien, sa at studien demonstrerte den første bruken av mikrofluidika for å styre iPSC-er til å bli nevrale stamceller.
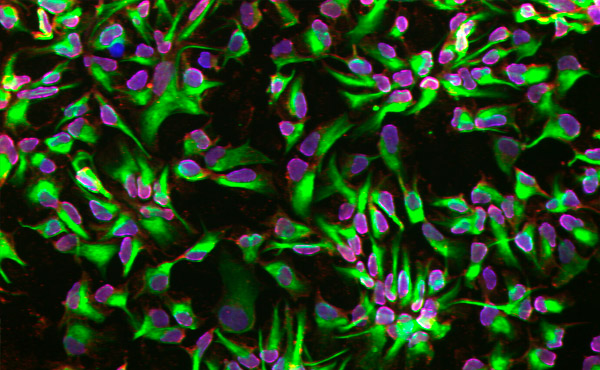
Nevrale stamceller differensierte ved hjelp av en mikrofluidisk plattform. Foto: KTH Royal Institute of Technology
Transformasjonen av normale celler til nevrale stamceller er faktisk en totrinnsprosess. Først blir cellene utsatt for biokjemiske signaler som får dem til å bli pluripotente stamceller (iPSC-er), som kan generere forskjellige typer celler.
Deretter overføres de til en kultur som etterligner signalene og utviklingsprosessene som er involvert i dannelsen av nervesystemet. Dette trinnet, kalt nevral differensiering, omdirigerer cellene mot å bli nevrale stamceller.
I løpet av det siste tiåret har laboratoriemiljøet for denne typen arbeid gradvis gått over fra tradisjonelle nettbrett til mikrofluidiske enheter. Herland sier at den nye plattformen representerer en forbedring innen mikrofluidikk for begge trinnene: iPSC-generering og differensiering av nevrale stamceller.
Ved å bruke celler fra biopsier fra menneskelig hud fant forskerne at den mikrofluidiske plattformen akselererte cellenes forpliktelse til en nevral skjebne på et tidligere stadium sammenlignet med de som differensierte i konvensjonelle plater.
«Vi har dokumentert at det begrensede miljøet på den mikrofluidiske plattformen forsterker forpliktelsen til å generere nevrale stamceller», sier Herland.
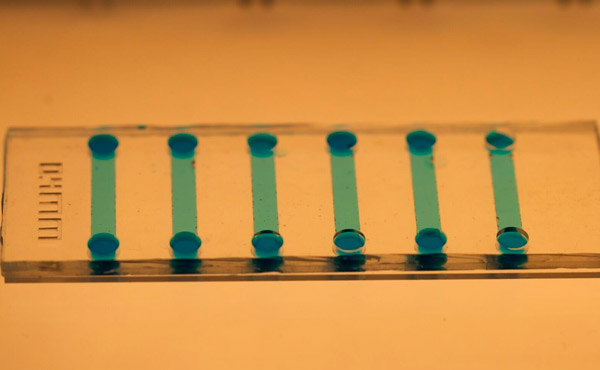
Et nærbilde av den mikrofluidiske brikken som brukes til å indusere stamceller. Foto: KTH Royal Institute of Technology
Jain sier at mikrofluidbrikken er enkel å lage ved hjelp av polydimetylsiloksan (PDMS), og den mikroskopiske størrelsen gir betydelige besparelser på reagenser og cellemateriale.
Plattformen kan enkelt modifiseres for å imøtekomme differensiering til andre celletyper, legger han til. Den kan automatiseres, noe som gir et lukket system som sikrer konsistens og pålitelighet i produksjonen av svært homogene cellepopulasjoner.
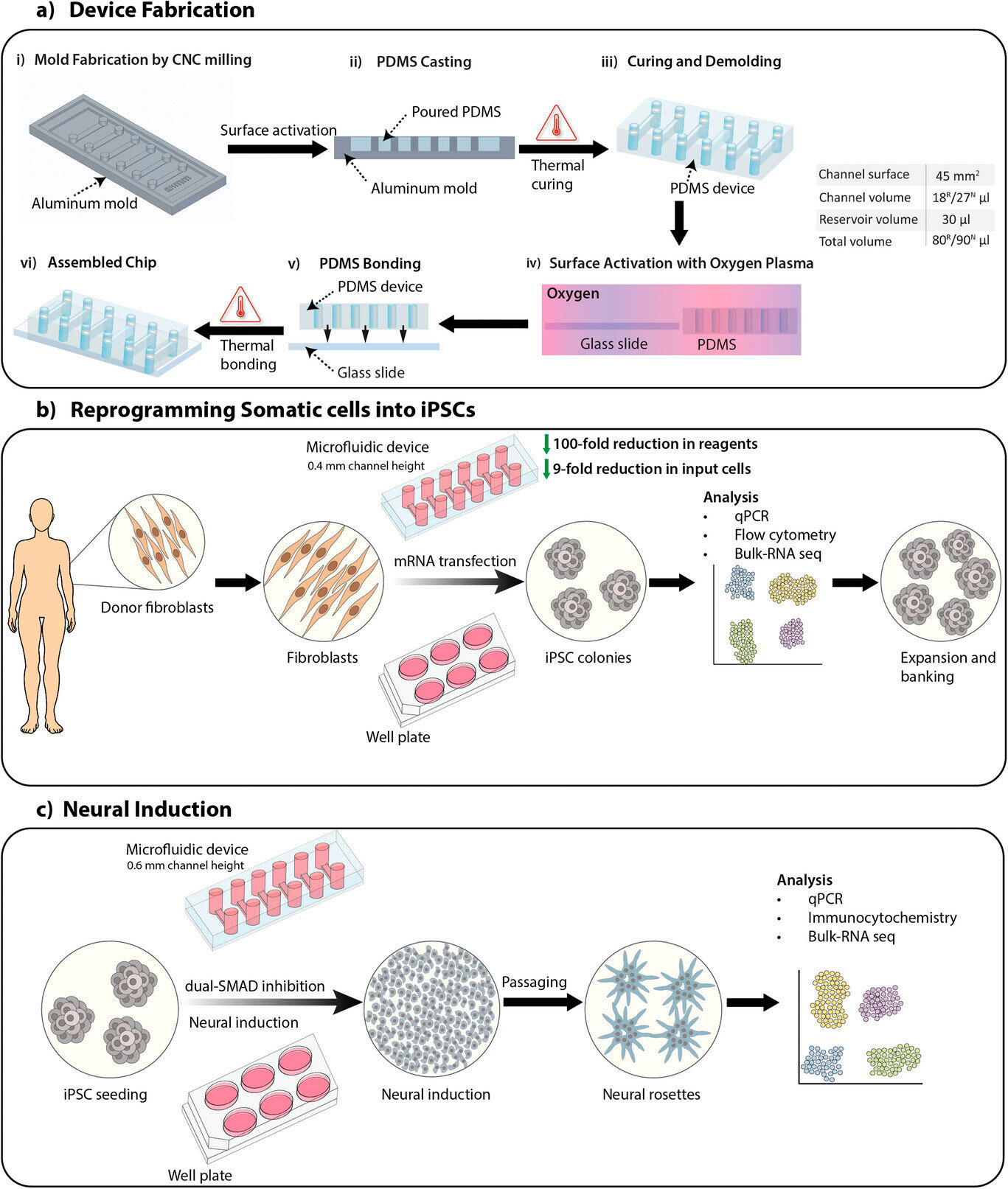
Oversikt over studien, inkludert fabrikasjon av enheter, omprogrammering av somatiske celler til induserte pluripotente stamceller (iPSC-er) og nevral induksjon av iPSC-er ved bruk av SMAD dobbel inhibisjonsprotokoll for å generere nevrale stamceller.
A) Fabrikasjonsprosess for en mikrofluidisk enhet med 0,4 mm og 0,6 mm høye kanaler for henholdsvis omprogrammering av somatiske celler (R) og nevral induksjon (N). Kanalvolum og totalt volum er listet opp i tabellen.
B) Oversikt over omprogrammeringsprosessen av somatiske celler til iPSC-er på mikrofluidiske enheter og plater ved bruk av mRNA-transfeksjon.
C) Oversikt over den nevrale induksjonsprosessen av iPSC-er til nevrale stamceller på mikrofluidiske enheter og plater ved bruk av SMAD dobbel inhibisjonsprotokoll.
Kilde: Advanced Science (2024). DOI: 10.1002/advs.202401859
«Dette er et skritt mot å gjøre personlig tilpassede celleterapier for Alzheimers og Parkinsons sykdommer tilgjengelige», legger Jain til.
Studien involverte også forskere fra Karolinska Institutet og Lunds universitet, som samarbeidet i IndiCell-konsortiet.
