Activation of innate immunity: an important part of the mechanism identified
Sist anmeldt: 14.06.2024

Alt iLive-innhold blir gjennomgått med medisin eller faktisk kontrollert for å sikre så mye faktuell nøyaktighet som mulig.
Vi har strenge retningslinjer for innkjøp og kun kobling til anerkjente medieområder, akademiske forskningsinstitusjoner og, når det er mulig, medisinsk peer-evaluerte studier. Merk at tallene i parenteser ([1], [2], etc.) er klikkbare koblinger til disse studiene.
Hvis du føler at noe av innholdet vårt er unøyaktig, utdatert eller ellers tvilsomt, velg det og trykk Ctrl + Enter.
LMU researchers have deciphered the complex interaction of various enzymes around the innate immune receptor Toll-like receptor 7 (TLR7), which plays an important role in protecting our bodies from viruses.
Toll-like receptor 7 (TLR7), located on the dendritic cells of our immune system, plays a critical role in our natural defense against viruses. TLR7 recognizes single-stranded viral and other foreign RNA and activates the release of inflammatory mediators. Dysfunction of this receptor also plays a key role in autoimmune diseases, making understanding and, ideally, modulation of the mechanism of TLR7 activation even more important.
Researchers led by Professor Veit Hornung and Marlin Berouti from the Center for Genetics Munich and the Department of Biochemistry at LMU were able to delve into the complex activation mechanism. It was known from previous studies that complex RNA molecules must be cut in order for the receptor to recognize them.
Using a range of technologies from cell biology to cryo-electron microscopy, LMU researchers have uncovered how single-stranded foreign RNA is processed to detect TLR7. Their work was published in Immunity magazine.
Numerous enzymes are involved in the recognition of foreign RNA
During evolution, the immune system specialized in recognizing pathogens by their genetic material. For example, the innate immune receptor TLR7 is stimulated by viral RNA. We can think of viral RNAs as long strands of molecules that are too large to be recognized as ligands for TLR7. This is where nucleases come to the rescue—molecular cutting tools that cut the “strand of RNA” into small pieces.
Endonucleases cut RNA molecules down the middle like scissors, while exonucleases cut the strand from one end to the other. This process generates different pieces of RNA that can now bind to two different pockets of the TLR7 receptor. Only when both receptor binding pockets are occupied by these pieces of RNA is a signaling cascade triggered, which activates the cell and causes an alarm state.
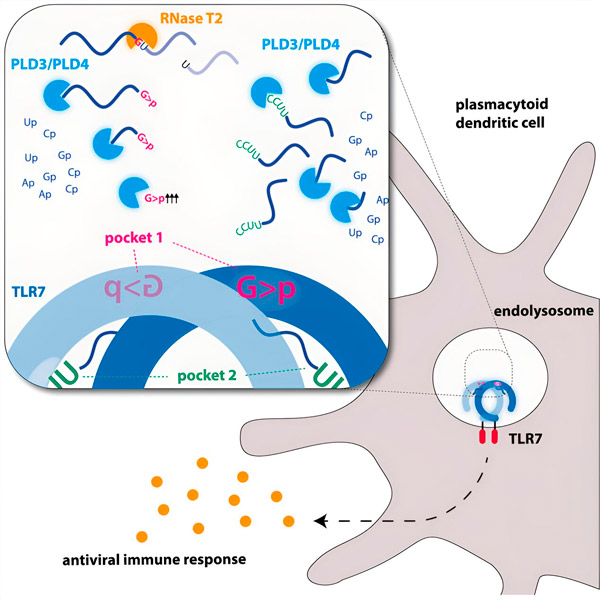
Graphic image. Source: Immunity (2024). DOI: 10.1016/j.immuni.2024.04.010
Researchers have discovered that TLR7 RNA recognition requires the activity of the endonuclease RNase T2, acting in conjunction with the exonucleases PLD3 and PLD4 (phospholipase D3 and D4). "Although it was known that these enzymes can degrade RNA," says Hornung, "we have now demonstrated that they interact and thereby activate TLR7."
Balancing the immune system
The researchers also discovered that PLD exonucleases play a dual role in immune cells. In the case of TLR7, they have a pro-inflammatory effect, whereas in the case of another TLR receptor, TLR9, they have an anti-inflammatory effect. "This dual role of PLD exonucleases suggests a finely coordinated balance to control proper immune responses," explains Berouti.
"The simultaneous stimulation and inhibition of inflammation by these enzymes may serve as an important protective mechanism to prevent dysfunction in the system." What role other enzymes may play in this signaling pathway and whether the molecules involved are suitable as targets for therapy will be the subject of further research.
