Researchers identify mutations that protect against B-cell cancer
Sist anmeldt: 14.06.2024

Alt iLive-innhold blir gjennomgått med medisin eller faktisk kontrollert for å sikre så mye faktuell nøyaktighet som mulig.
Vi har strenge retningslinjer for innkjøp og kun kobling til anerkjente medieområder, akademiske forskningsinstitusjoner og, når det er mulig, medisinsk peer-evaluerte studier. Merk at tallene i parenteser ([1], [2], etc.) er klikkbare koblinger til disse studiene.
Hvis du føler at noe av innholdet vårt er unøyaktig, utdatert eller ellers tvilsomt, velg det og trykk Ctrl + Enter.
Researchers at the University of Texas Southwestern Medical Center were able to suppress leukemia and lymphoma in a mouse model genetically predisposed to these cancers by completely or partially depleting a protein called midnoline in B cells.
Their findings, published in the Journal of Experimental Medicine, could lead to the development of new treatments for these diseases that avoid the serious side effects of current therapies.
“We used a purely genetic method to find a drug target, and this target turned out to be sensational because B-cell leukemias and lymphomas are highly dependent on it, while most host tissues are not,” said study leader Bruce Beutler, MD, is director of the Center for Genetic Host Defense and professor of immunology and internal medicine at the University of Texas Southwestern Medical Center.
Dr. Bütler, who received the Nobel Prize in Physiology or Medicine in 2011 for his discovery of an important group of pathogen sensors known as Toll-like receptors found on immune cells, has long used mutagenesis—the introduction of mutations into the genes of animal models by exposing them to chemicals. A substance called N-ethyl-N-nitrosourea (ENU) as a key tool for studying gene function.
Beutler's laboratory recently developed a method known as automated meiotic mapping (AMM), which traces unusual features in mutant mice back to causative mutations, thereby identifying genes essential for maintaining normal physiology.
Mutagenesis often causes the development of genetic diseases in animals, which provides insight into the function of the affected genes by studying the abnormalities in animals. However, as Dr. Beutler explained, mutations can also provide protection against disease.
Examples include mutations that protect people with HIV or people with hereditary sickle cell disease from developing symptoms. The mechanisms underlying some protective mutations have inspired the development of drugs to treat various diseases.
Looking for protective mutations for immune disorders, researchers tested mutant mice for immune cells with unusual features. In several sets of animals with unusually low numbers of B cells—an important component of the adaptive immune system responsible for producing antibodies—the researchers used AMM to trace this deficiency to mutations in mynoline, a protein found primarily in B cells.
Although animals completely lacking midnolin die during development before birth, milder mutations, including some introduced through genetic techniques that remove the gene in adulthood, have caused no apparent harm.
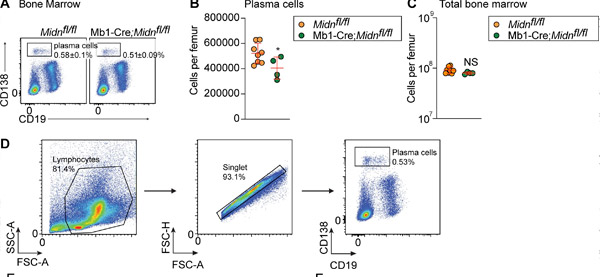
Plasma cell production after immunization with TD antigen β-galactosidase in Mb1-Cre;Midn fl/fl mice. (A and B) Representative flow cytometry plots of (A) and number (B) of plasma cells in the bone marrow of 8-week-old Mb1-Cre;Midn fl/fl and Midn fl/fl mice after immunization with β-galactosidase. (C) Total number of bone marrow cells per femur. (D) Plasma cell isolation strategy. Source: Journal of Experimental Medicine (2024). DOI: 10.1084/jem.20232132
Researchers significantly reduced or eliminated midnoline in mice genetically predisposed to B-cell leukemias and lymphomas, cancers in which B cells divide uncontrollably. Although mice with normal levels of midnoline died from these diseases by 5 months, most of those with less or no midnoline never developed malignant tumors.
Additional experiments showed that midnoline's role in B cells is to stimulate the activity of proteasomes, cellular organelles that recycle damaged or no longer needed proteins. Some therapies currently used to treat B-cell leukemias and lymphomas work by inhibiting proteasome activity, much as midnoline removal does, Dr. Beutler explained.
However, unlike these drugs, which have many potentially serious side effects, eliminating or reducing midnoline in animal models did not appear to have negative consequences.
Future research will focus on developing drugs that inhibit midnoline, which could ultimately provide the basis for new treatments for B-cell cancers.
