Nye publikasjoner
Lukket sløyfe kan forbedre leveringen av cellegift
Sist anmeldt: 02.07.2025

Alt iLive-innhold blir gjennomgått med medisin eller faktisk kontrollert for å sikre så mye faktuell nøyaktighet som mulig.
Vi har strenge retningslinjer for innkjøp og kun kobling til anerkjente medieområder, akademiske forskningsinstitusjoner og, når det er mulig, medisinsk peer-evaluerte studier. Merk at tallene i parenteser ([1], [2], etc.) er klikkbare koblinger til disse studiene.
Hvis du føler at noe av innholdet vårt er unøyaktig, utdatert eller ellers tvilsomt, velg det og trykk Ctrl + Enter.
Når kreftpasienter gjennomgår cellegiftbehandling, beregnes dosene av de fleste legemidler basert på pasientens kroppsoverflateareal. Dette estimeres ved hjelp av en ligning som tar hensyn til pasientens høyde og vekt. Denne ligningen ble formulert i 1916 basert på data fra bare ni pasienter.
Denne forenklede doseringsmetoden tar ikke hensyn til andre faktorer og kan føre til at en pasient får for mye eller for lite av et legemiddel. Som et resultat kan noen pasienter oppleve unødvendig toksisitet eller utilstrekkelig effekt av cellegiften de får.
For å forbedre nøyaktigheten av cellegiftdoseringen har MIT-ingeniører utviklet en alternativ tilnærming som gjør det mulig å tilpasse dosen til hver pasient. Systemet deres måler mengden legemiddel i pasientens kropp og sender disse dataene til en kontroller som kan justere infusjonshastigheten deretter.
Denne tilnærmingen kan bidra til å kompensere for forskjeller i legemiddelfarmakokinetikk forårsaket av kroppssammensetning, genetisk predisposisjon, cellegiftindusert organtoksisitet, interaksjoner med andre legemidler og mat, og døgnrytmevariasjoner i enzymene som er ansvarlige for å bryte ned cellegiftmedisiner, sier forskerne.
«Ved å anerkjenne fremskritt i forståelsen av hvordan legemidler metaboliseres og bruke tekniske verktøy for å forenkle personlig dosering, tror vi at vi kan bidra til å forvandle sikkerheten og effektiviteten til mange legemidler», sa Giovanni Traverso, førsteamanuensis i maskinteknikk ved MIT, gastroenterolog ved Brigham and Women's Hospital og seniorforfatter av studien.
Louis DeRidder, en masterstudent ved MIT, er hovedforfatter av artikkelen som er publisert i tidsskriftet Med.
Kontinuerlig overvåking
I denne studien fokuserte forskerne på et legemiddel kalt 5-fluorouracil, som brukes til å behandle kolorektal kreft og andre kreftformer. Legemidlet administreres vanligvis over en 46-timers periode, og doseringen bestemmes ved hjelp av en formel basert på pasientens høyde og vekt, som gir et estimat av kroppsoverflateareal.
Denne tilnærmingen tar imidlertid ikke hensyn til forskjeller i kroppssammensetning som kan påvirke hvordan legemidlet fordeles i kroppen, eller genetiske variasjoner som påvirker hvordan det metaboliseres. Disse forskjellene kan føre til skadelige bivirkninger hvis det gis for mye av legemidlet. Hvis det ikke gis nok av legemidlet, kan det hende at det ikke dreper svulsten som forventet.
«Folk med samme kroppsoverflate kan ha svært ulik høyde og vekt, ulik muskelmasse eller ulik genetikk, men så lenge høyden og vekten som er koblet til den ligningen gir samme kroppsoverflate, er dosen deres identisk», sier DeRidder, en doktorgradskandidat i medisinsk ingeniørfag og medisinsk fysikkprogram ved Harvard-MIT-programmet i helsevitenskap og teknologi.
En annen faktor som kan endre mengden legemiddel i blodet til enhver tid er døgnrytmevariasjoner i et enzym kalt dihydropyrimidindehydrogenase (DPD), som bryter ned 5-fluorouracil. Ekspresjonen av DPD, som mange andre enzymer i kroppen, reguleres av en døgnrytme. Dermed er nedbrytningen av 5-FU av DPD ikke konstant, men varierer med tidspunktet på døgnet. Disse døgnrytmene kan resultere i en tidobling i mengden 5-FU i en pasients blod under en infusjon.
«Ved å bruke kroppsoverflateareal for å beregne cellegiftdosen, vet vi at to personer kan ha svært ulik toksisitet fra 5-fluorouracil. Én pasient kan ha behandlingssykluser med minimal toksisitet, og deretter en syklus med forferdelig toksisitet. Noe har endret seg i måten pasienten metaboliserte cellegiften fra en syklus til den neste. Vår utdaterte doseringsmetode fanger ikke opp disse endringene, og pasientene lider som følge av dette», sier Douglas Rubinson, en klinisk onkolog ved Dana-Farber Cancer Institute og en av forfatterne av artikkelen.
En måte å forsøke å kompensere for variasjon i farmakokinetikken til cellegiftbehandling er en strategi som kalles terapeutisk legemiddelmonitorering, der pasienten avgir en blodprøve på slutten av en behandlingssyklus. Etter at denne prøven er analysert for legemiddelkonsentrasjoner, kan doseringen justeres om nødvendig i begynnelsen av neste syklus (vanligvis to uker for 5-fluorouracil).
Denne tilnærmingen har vist seg å føre til bedre resultater for pasienter, men har ikke blitt mye brukt for cellegiftbehandlinger som 5-fluorouracil.
MIT-forskerne ønsket å utvikle en lignende type overvåking, men på en automatisert måte som ville tillate personlig dosering av legemidler i sanntid, noe som kunne føre til bedre resultater for pasientene.
I deres lukkede system kan legemiddelkonsentrasjoner overvåkes kontinuerlig, og denne informasjonen brukes til å automatisk justere infusjonshastigheten til cellegiften for å holde dosen innenfor målområdet.
Dette lukkede systemet gjør det mulig å tilpasse medikamentdosering for å ta hensyn til døgnrytmer i nivåene av medikamentmetaboliserende enzymer, samt eventuelle endringer i pasientens farmakokinetikk siden siste behandling, for eksempel cellegiftindusert organtoksisitet.
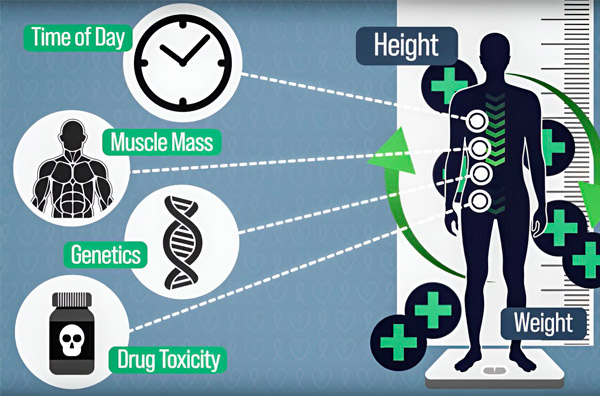
For å gjøre cellegiftdoseringen mer presis har MIT-ingeniører utviklet en måte å kontinuerlig måle mengden legemiddel i en pasients kropp i løpet av en infusjon som varer flere timer. Dette vil bidra til å kompensere for forskjeller forårsaket av kroppssammensetning, genetikk, legemiddeltoksisitet og døgnrytmevariasjoner. Kilde: Med tillatelse fra forskerne.
Det nye systemet som forskerne har utviklet, kjent som CLAUDIA (Closed-Loop AUtomated Drug Infusion regulator), bruker kommersielt tilgjengelig utstyr for hvert trinn. Blodprøver tas hvert femte minutt og klargjøres raskt for analyse. Konsentrasjonen av 5-fluorouracil i blodet måles og sammenlignes med målområdet.
Differansen mellom målkonsentrasjonen og den målte konsentrasjonen legges inn i en kontrollalgoritme, som deretter justerer infusjonshastigheten etter behov for å opprettholde dosen innenfor konsentrasjonsområdet der legemidlet er effektivt og ikke-giftig.
«Vi har utviklet et system der vi kontinuerlig kan måle legemiddelkonsentrasjonen og justere infusjonshastigheten deretter for å opprettholde legemiddelkonsentrasjonen innenfor det terapeutiske vinduet», sier DeRidder.
Rask justering
I dyreforsøk fant forskerne ut at de ved bruk av CLAUDIA kunne holde mengden legemiddel som sirkulerte i kroppen innenfor målområdet omtrent 45 prosent av tiden.
Legemiddelnivåene hos dyr som fikk cellegift uten CLAUDIA forble innenfor målområdet i gjennomsnitt bare 13 prosent av tiden. Forskerne testet ikke effektiviteten av legemiddelnivåene i denne studien, men det antas at det å opprettholde konsentrasjoner innenfor målområdet gir bedre resultater og mindre toksisitet.
CLAUDIA klarte også å opprettholde 5-fluorouracil-dosen innenfor målområdet selv når et legemiddel som hemmer DPD-enzymet ble administrert. Hos dyr som fikk denne hemmeren uten kontinuerlig overvåking og justering, økte 5-fluorouracil-nivåene opptil åtte ganger.
For denne demonstrasjonen utførte forskerne manuelt hvert trinn i prosessen ved hjelp av standardutstyr, men planlegger nå å automatisere hvert trinn slik at overvåking og dosejusteringer kan gjøres uten menneskelig inngripen.
For å måle legemiddelkonsentrasjoner brukte forskerne høytrykksvæskekromatografi-massespektrometri (HPLC-MS), en teknikk som kan tilpasses for å oppdage nesten alle typer legemidler.
«Vi ser en fremtid der vi kan bruke CLAUDIA for ethvert legemiddel som har de riktige farmakokinetiske egenskapene og er detekterbart ved HPLC-MS, noe som muliggjør personlig dosering for mange forskjellige legemidler», sier DeRidder.
