Nye publikasjoner
Behandling med biskjoldbruskkjertelhormoner bidrar til å bremse utviklingen av artrose
Sist anmeldt: 02.07.2025

Alt iLive-innhold blir gjennomgått med medisin eller faktisk kontrollert for å sikre så mye faktuell nøyaktighet som mulig.
Vi har strenge retningslinjer for innkjøp og kun kobling til anerkjente medieområder, akademiske forskningsinstitusjoner og, når det er mulig, medisinsk peer-evaluerte studier. Merk at tallene i parenteser ([1], [2], etc.) er klikkbare koblinger til disse studiene.
Hvis du føler at noe av innholdet vårt er unøyaktig, utdatert eller ellers tvilsomt, velg det og trykk Ctrl + Enter.

Cornell-forskere har funnet ut at forbehandling med parathyroidhormon, som ofte brukes for å øke benmassen ved osteoporose, kan bidra til å forbedre bruskhelsen og bremse utviklingen av slitasjegikt.
Teamet, ledet av Marjolijn van der Meulen, direktør for James M. og Marsha McCormick School of Biomedical Engineering ved Cornell's College of Engineering, identifiserte også genuttrykkssignaturer som potensielt kan brukes til tidlig oppdagelse av degenerativ leddsykdom.
Resultatene ble publisert i tidsskriftet Science Advances. Artikkelens medforfattere er Adrien Antoinette og Sofia Zimyan.
Van der Meulen spesialiserer seg på å studere mekanikkens rolle i skjelettet og hvordan muskel- og skjelettsystemet – bein, brusk, ledd – reagerer på belastning ved hjelp av vektbærende og kompresjonsteknikker på leggen og kneleddet.
Belastning har sine fordeler og ulemper. Det øker benmassen og kan brukes som behandling for osteoporose. Samtidig skader belastning også brusken i leddene, på samme måte som degenerasjonen man ser ved slitasjegikt. Van der Meulen og laboratoriet hennes fokuserer i økende grad på hvilken rolle bein spiller i utviklingen av leddskade.
I den nye studien gjennomførte teamet en totrinnsprosess. Først behandlet de mus daglig med parathyroidhormon, et legemiddel foreskrevet mot osteoporose, for å øke benmassen i åtte uker. I den andre fasen påførte teamet daglig vektbærende stress på musenes skinneben og brukte et annet osteoporoselegemiddel, alendronat, som effektivt slår av beinets evne til å reparere seg selv (remodellere) i seks uker.
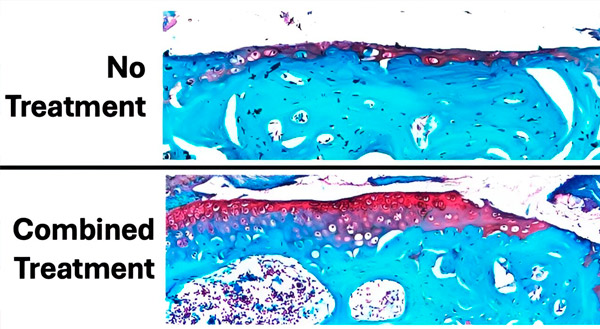
Figuren viser omfanget av bruskskade etter 6 uker med daglig belastning og behandling sammenlignet med et kontrollkne uten belastning og uten bruskskade. Brusken er farget rød og beinet er blågrønt. Totalt sett viste forbehandling med parathyroidhormon før belastning og behandling med alendronat under belastning minst mulig bruskskade (tap av rødfarget vev) og best bevaring av brusk. Kilde: Science Advances (2024). DOI: 10.1126/sciadv.adk8402
Forskerne fant at parathyroidhormon direkte forbedret bruskshelsen og bremset utviklingen av skade, mens alendronat reduserte subkondrale beinforandringer forbundet med slitasjegikt.
«Selv etter seks uker med skade var effekten av den åtte uker lange forbehandlingen fortsatt betydelig. PTH gjorde mer enn bare å øke benmassen, fordi det viser seg at det også påvirker brusk», sa van der Meulen. «Musenes knær hadde tykkere brusk etter åtte uker, noe som var uventet. Tykkere brusk beskytter sannsynligvis mot ytterligere leddskade.»
Teamet gjentok eksperimentet og brukte transkriptomikk til å analysere genuttrykk i RNA ekstrahert fra brusk, bein og lymfeknuter hos mus. Leddskader gjenspeiles i tidlige transkriptomiske endringer, og begge behandlingene sammen førte til tidlig modulering av immunsignalering.
"Genuttrykk viste at begge legemidlene sammen hadde størst effekt i å redusere uttrykket av gener assosiert med bruskskade, spesielt ved å endre uttrykket av immungener," sa Zimyan.
Det neste trinnet er å avgjøre om behandling med parathyroidhormon kan bremse eller til og med reversere utviklingen av slitasjegikt når den først har oppstått, og å bruke gensignaturer til å utvikle tidlig diagnostikk for sykdommen.
«Disse resultatene tyder på at disse behandlingene også kan være nyttige for mennesker. Og den gode nyheten er at disse behandlingene allerede er godkjent av FDA, men ikke for denne bruken», sa van der Meulen.
