Nye publikasjoner
Et nytt perspektiv på nevrodegenerasjon: rollen til det nevrokjemiske stoffet T14 ved Alzheimers sykdom
Last reviewed: 02.07.2025

Alt iLive-innhold blir gjennomgått med medisin eller faktisk kontrollert for å sikre så mye faktuell nøyaktighet som mulig.
Vi har strenge retningslinjer for innkjøp og kun kobling til anerkjente medieområder, akademiske forskningsinstitusjoner og, når det er mulig, medisinsk peer-evaluerte studier. Merk at tallene i parenteser ([1], [2], etc.) er klikkbare koblinger til disse studiene.
Hvis du føler at noe av innholdet vårt er unøyaktig, utdatert eller ellers tvilsomt, velg det og trykk Ctrl + Enter.
Et internasjonalt team av klinikere og nevroforskere har publisert en ny oversiktsartikkel om prosessen med nevrodegenerasjon. Funnene deres utforsker mekanismen som går forut for amyloiddannelse, inkludert et viktig nevrokjemisk stoff som fremmer prosessen.
Artikkelen, publisert i tidsskriftet Alzheimer's & Dementia, fokuserer på isodendrittkjerner, en gruppe nevroner som er forskjellige fra andre hjerneceller og tidligere har blitt identifisert som spesielt sårbare ved Alzheimers sykdom (AD).
Forfatterne erkjenner at amyloid er en betydelig faktor i Alzheimers sykdom i sent stadium, men bemerker at det er fraværende i disse nevronene tidlig. Hvis det oppstår skade på disse sårbare nevronene i voksen alder, reagerer de ved å mobilisere en responsmekanisme. Denne mekanismen fremmer vanligvis nevronvekst i fosterlivet og tidlig i livet, men er skadelig i voksen alder.
Gjennomgangen beskriver hvordan nøkkelmolekylet som driver denne prosessen er det bioaktive 14-mer-peptidet T14, som selektivt aktiverer én målreseptor. I den modne hjernen forårsaker T14 nevrondød og starter en negativ snøball som blir sterkere over tid, i stedet for å gjenopprette normal funksjon.
Isodendrittkjernene, som ligger dypt i hjernen, er ansvarlige for oppvåkning og søvn/våken-sykluser, og er ikke direkte knyttet til høyere funksjoner som hukommelse. Dermed kan degenerasjonsprosessen fortsette uten åpenbare symptomer inntil skaden sprer seg til områder som er ansvarlige for kognisjon.
Forklaringen som foreslås i artikkelen kan forklare den lange forsinkelsen på 10–20 år fra nervesystemets tap begynner å utvikle seg til kognitiv svikt begynner.
Gjennomgangen rapporterer at T14 kan oppdages på et svært tidlig stadium av Alzheimers sykdom, noe som kan tjene som en presymptomatisk indikasjon på begynnelsen av nevrodegenerasjon og dermed kan utvikles som en biomarkør.
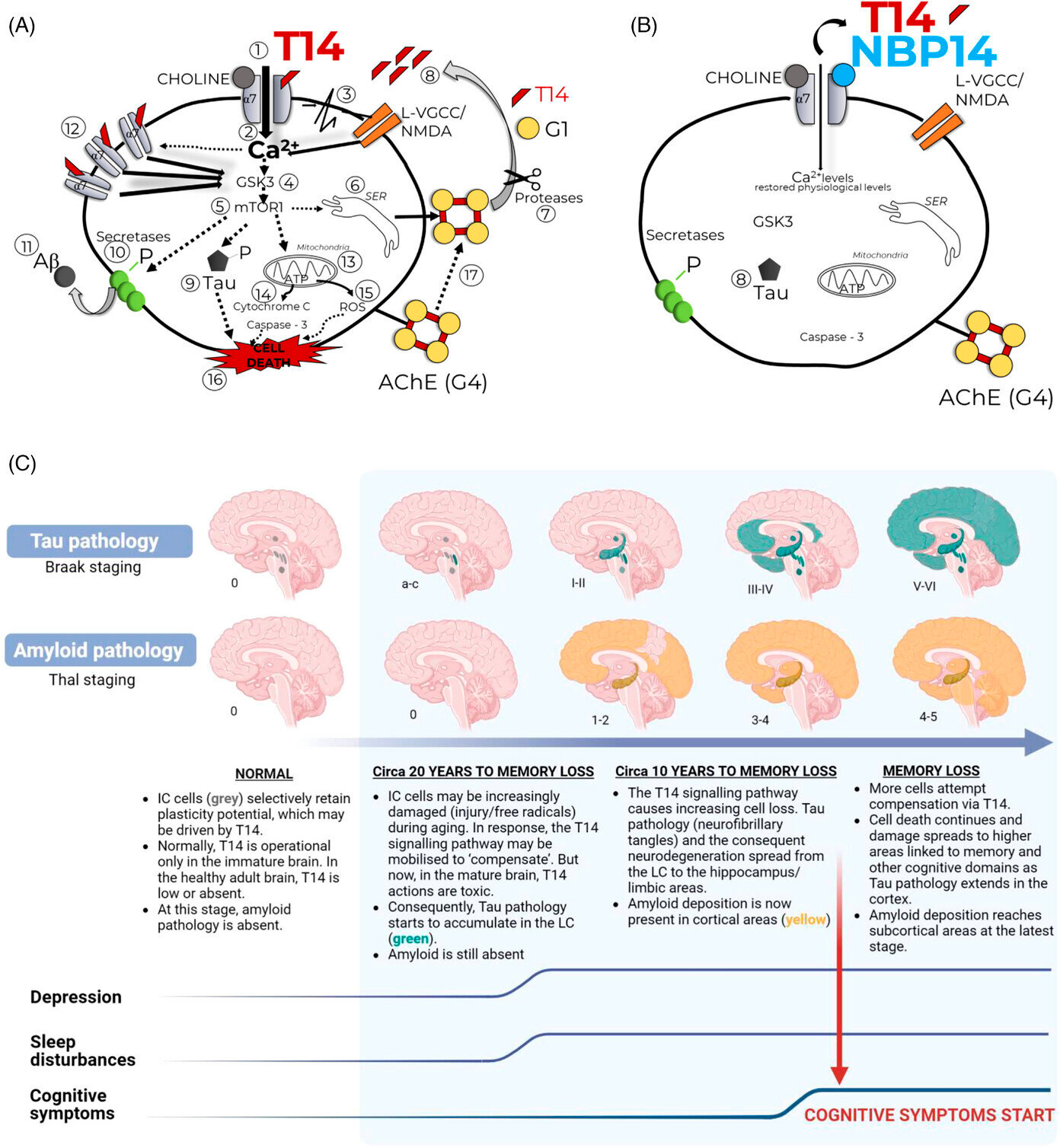
I tillegg beskriver forfatterne hvordan en syklisert versjon av T14, NBP14, kan blokkere virkningen av T14. NBP14 har vist seg å forhindre hukommelsessvekkelse i en musemodell av AD, og virkningsmekanismen har blitt demonstrert i en rekke studier, inkludert obduksjoner av menneskelig hjernevev. Dermed kan NBP14 danne grunnlaget for en ny terapeutisk strategi.
Denne nye tilnærmingen gir viktige oppdagelser som kan ha betydelig innvirkning på tidlig diagnose og behandling av Alzheimers sykdom, og understreker viktigheten av videre forskning på dette området.
