Nye publikasjoner
LM11A-31 bremser utviklingen av Alzheimers sykdom i studie
Sist anmeldt: 02.07.2025

Alt iLive-innhold blir gjennomgått med medisin eller faktisk kontrollert for å sikre så mye faktuell nøyaktighet som mulig.
Vi har strenge retningslinjer for innkjøp og kun kobling til anerkjente medieområder, akademiske forskningsinstitusjoner og, når det er mulig, medisinsk peer-evaluerte studier. Merk at tallene i parenteser ([1], [2], etc.) er klikkbare koblinger til disse studiene.
Hvis du føler at noe av innholdet vårt er unøyaktig, utdatert eller ellers tvilsomt, velg det og trykk Ctrl + Enter.

I en nylig studie publisert i tidsskriftet Nature Medicine, gjennomførte forskere en randomisert, dobbeltblind, placebokontrollert fase 2a-studie for å undersøke sikkerheten og effekten av LM11A-31 i behandlingen av Alzheimers sykdom (AD) gjennom modulering av p75-nevrotrofinreseptoren (p75NTR).
Sen debut av Alzheimers sykdom er den vanligste formen for demens, karakterisert ved synaptisk svikt, degenerasjon og tap av nerveceller. Selv om de to ledende legemidlene for behandling av Alzheimers sykdom retter seg mot akkumulering av unormale amyloid-β- eller tau-proteiner, adresserer de bare deler av patofysiologien. En annen tilnærming involverer målretting av reseptorer og signalnettverk som påvirker grunnleggende biologiske veier. Prekliniske studier viser at modulering av p75NTR med et nytt lite kjemisk molekyl, LM11A-31, reduserer synaptisk tap forårsaket av amyloid og unormal tau.
Beskrivelse av studien
I denne randomiserte kliniske studien undersøkte forskerne om LM11A-31 kunne bremse utviklingen av Alzheimers sykdom ved å modulere p75NTR hos mennesker.
Studiedeltakerne fikk LM11A-31 orale kapsler i doser på 200 mg og 400 mg eller placebo i forholdet 1:1:1 til 242 pasienter med mild til moderat astma i 26 uker. Deltakerne hadde biologisk bekreftet Alzheimers sykdom (nivå av amyloid β-protein 42 (Aβ42) i cerebrospinalvæsken under 550 ng/L eller Aβ42:β40-forhold under 0,89) diagnostisert i henhold til McKhann-kriterier, med Mini-Psychiatric Examination (MMSE)-skårer på 18 til 26, Geriatric Depression Scale (GDS)-skårer under 5,0, modifisert Hachinski Ischemic Scale (HIS)-skårer ≤ 4,0, formell utdanning ≥ 8 år og tidligere kognitiv nedgang ≥ 6 måneder.
Kvalifiserte deltakere hadde tatt acetylkolinesterasehemmere (AChEI-er) eller partielle NMDA-reseptorantagonister i ≥ 3 måneder før studiestart. De tok ikke illegale legemidler som antipsykotika, benzodiazepiner, antiepileptika, beroligende midler, sentralt aktive antihypertensiva, nootropika (unntatt ginkgo biloba) eller opioidholdige smertestillende midler.
Studiens primære utfall var sikkerhet og toleranse, vurdert ved hjelp av Columbia Suicidal Thoughts and Behavior Severity Rating Scale (C-SSRS), vitale tegn, blodtrykk og hematologiske parametere. Strukturell magnetisk resonansavbildning (cMRI), fluorodeoksyglukose positronemisjonstomografi (FDG-PET) og biomarkører for cerebrospinalvæske (CSF) ble brukt til å vurdere sekundære kognitive utfall. AD-mål inkluderte Thr181-fosforylert tau, totalt tau-protein, Aβ40, Aβ42 og AChE-aktivitet. Teamet brukte en tilpasset nevropsykologisk test for å vurdere sekundære kognitive utfall ved baseline, uke 12 og 26.
Forskningsresultater
Studien fant at LM11A-31 var trygt og godt tolerert, uten vesentlige sikkerhetsproblemer. De vanligste bivirkningene inkluderte hodepine, diaré, eosinofili og nasofaryngitt, med mage-tarmproblemer og eosinofili som de viktigste årsakene til seponering. Det var flere seponeringer i 400 mg-gruppen sammenlignet med 200 mg- og placebogruppene. MR avdekket ingen sikkerhetsproblemer, inkludert amyloidrelaterte abnormiteter. Det var ingen signifikante forskjeller i kognitive skårer eller amyloidabnormaliteter mellom de to behandlingsgruppene.
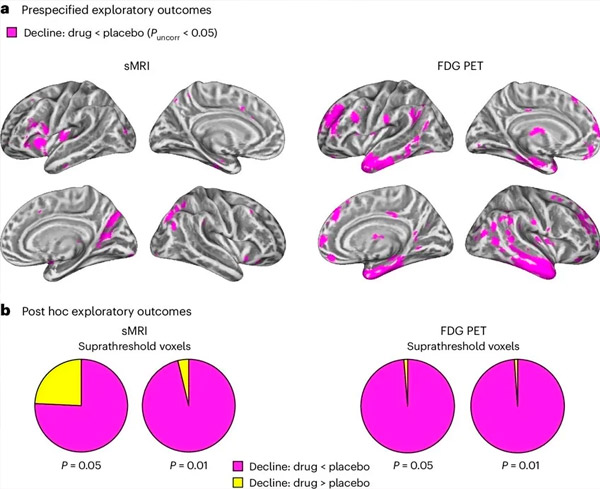
A. Toveis blandede modellanalyse av kovarians undersøkte interaksjoner mellom behandling (legemiddel eller placebo) og tid (før eller etter behandling). En ensidig t-kontrast som undersøkte interaksjonshypotesen (legemiddel bremser progresjon sammenlignet med placebo) viste at LM11A-31-behandling bremset longitudinell degenerasjon (venstre panel) og glukosehypometabolisme (høyre panel) i legemiddelgruppen (cMRI, n = 127; PET, n = 121) sammenlignet med placebogruppen (cMRI, n = 66; PET, n = 62). Voksler som viser denne interaksjonen er vist ved en ukorrigert terskel på P < 0,05 (magenta) på en populasjonsspesifikk kortikal overflate. Venstre og høyre hjernehalvdel er vist i henholdsvis øverste og nederste rad. Hjerneområder som viser interaksjoner som ikke er i samsvar med hypotesen er vist i figur 7 i tilleggsdataene.
B. Totalt antall vokseler i forhåndsdefinerte sårbare AD-hjerneområder (totalt areal av sektordiagrammer) som enten viser en interaksjon i tråd med hypotesen (magenta) eller en interaksjon som er inkonsistent med hypotesen (gul) i hver avbildningsmodalitet (cMRI, venstre panel; FDG PET, høyre panel) ved stadig mer liberale terskler av ukorrigert P < 0,01 og P < 0,05. Monte Carlo-simuleringer viste at forholdstallene mellom vokseler som viste effekter i tråd med hypotesen kontra inkonsistente med hypotesen var betydelig høyere enn de som ble observert basert på tilfeldig genererte data for både cMRI og PET (P < 0,001 for hver modalitet; tosidig test).
LM11A-31 reduserte effektivt økningen i CSF Aβ42 og Aβ40 sammenlignet med placebogruppen. Legemidlet viste også en reduksjon i median årlig prosentvis endring i den presynaptiske proteinbiomarkøren SNAP25 og en reduksjon i den postsynaptiske biomarkøren NG, noe som indikerer en nedgang i tapet av presynaptiske og postsynaptiske forbindelser. LM11A-31 reduserte også økningen i YKL40, noe som førte til en reduksjon i MMSE-score og en økning i ADAS-Cog-13-score. Legemidlet reduserte også tap av grå substans i frontallappen og bakre parietalcortex og en reduksjon i glukosemetabolismen i områder som entorhinal cortex, temporal cortex, hippocampus, insular cortex og prefrontal cortex.
Konklusjon
Studien konkluderte med at modulering av p75NTR av LM11A-31 er egnet for større kliniske studier. LM11A-31 oppfylte det primære sikkerhetsendepunktet og ble godt tolerert hos pasienter med mild til alvorlig Alzheimers sykdom. Resultatene indikerer behov for ytterligere studier med lengre behandlingsvarigheter for å evaluere potensialet til små molekyler til å regulere p75NTR som en sykdomsmodifiserende terapi ved Alzheimers sykdom. Studien viste at LM11A-31 signifikant påvirket flere biomarkører, inkludert Aβ40, Aβ42, SNAP25, NG og YKL40, noe som indikerer en avmatning av patologisk progresjon. Fremtidige studier kan evaluere ytterligere indikatorer på glialhelse.
