Nye publikasjoner
Avklaring av de cellulære mekanismene bak periodontitt ved hjelp av en forbedret dyremodell
Sist anmeldt: 02.07.2025

Alt iLive-innhold blir gjennomgått med medisin eller faktisk kontrollert for å sikre så mye faktuell nøyaktighet som mulig.
Vi har strenge retningslinjer for innkjøp og kun kobling til anerkjente medieområder, akademiske forskningsinstitusjoner og, når det er mulig, medisinsk peer-evaluerte studier. Merk at tallene i parenteser ([1], [2], etc.) er klikkbare koblinger til disse studiene.
Hvis du føler at noe av innholdet vårt er unøyaktig, utdatert eller ellers tvilsomt, velg det og trykk Ctrl + Enter.
Forskere fra Tokyo Medical and Dental University (TMDU) har utviklet en teknikk som muliggjør detaljert analyse av utviklingen av periodontitt over tid.
Periodontal sykdom, kjent som periodontitt, er den viktigste årsaken til tanntap og rammer nesten én av fem voksne over hele verden. I de fleste tilfeller skyldes tilstanden en inflammatorisk respons på en bakteriell infeksjon i vevet rundt tennene.
Etter hvert som tilstanden forverres, begynner tannkjøttet å trekke seg tilbake, og tennenes og beinets røtter blir synlige. Det er verdt å merke seg at forekomsten av periodontitt øker med alderen, og siden verdens befolkning lever lenger, er det viktig å ha en solid forståelse av de underliggende årsakene og utviklingen.
I en studie publisert i tidsskriftet Nature Communications fant forskere fra TMDU en måte å oppnå dette målet på ved å forbedre en mye brukt dyremodell for å studere periodontitt.
Det er vanskelig å studere periodontitt direkte hos mennesker. Som et resultat vender forskere seg ofte til dyremodeller for prekliniske studier. For eksempel har den "ligaturinduserte periodontitt-musemodellen" gjort det mulig for forskere å studere de cellulære mekanismene som ligger til grunn for tilstanden siden introduksjonen i 2012.
Enkelt sagt induserer denne modellen kunstig periodontal sykdom ved å plassere silketråder på jekslene til mus, noe som forårsaker plakkopphopning. Selv om denne metoden er praktisk og effektiv, fanger den ikke opp hele bildet av periodontitt.
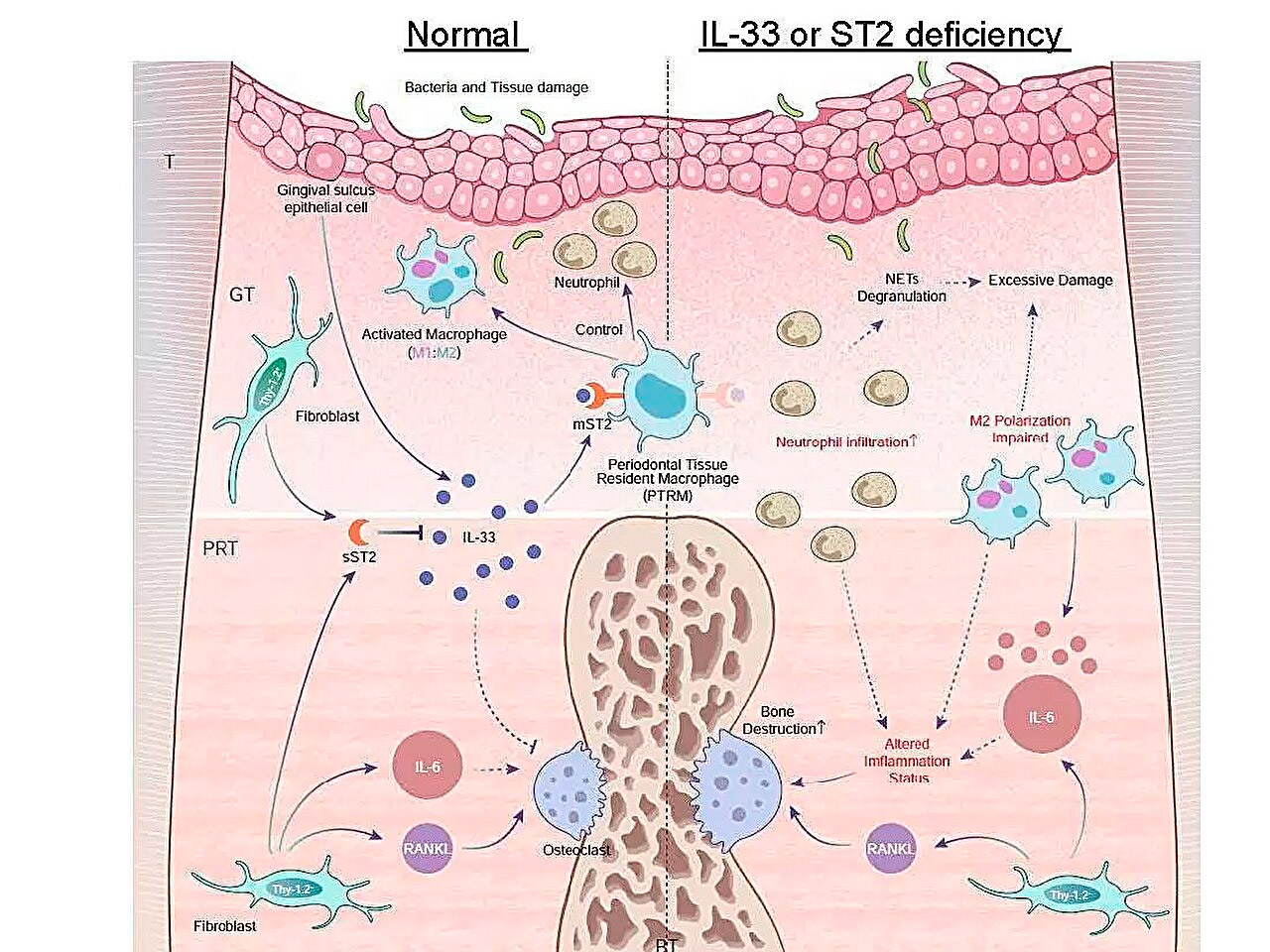
Skjematisk illustrasjon av inflammatoriske genuttrykksprofiler under periodontitt og rollen til IL-33/ST2-aksen i bekjempelse av akutt betennelse. Kilde: Tokyo Medical and Dental University.
«Selv om periodontalt vev består av gingiva, periodontalligament, alveolært bein og sement, utføres analysen vanligvis utelukkende på gingivaprøver på grunn av tekniske og kvantitative begrensninger», bemerker hovedforfatter av studien, Anhao Liu. «Denne prøvetakingsstrategien begrenser konklusjonene som kan trekkes fra disse studiene, så det er behov for metoder som tillater samtidig analyse av alle vevskomponenter.»
For å håndtere denne begrensningen utviklet forskerteamet en modifisert ligaturindusert periodontittmodell. I stedet for den klassiske enkeltligaturen brukte de en trippelligatur på øvre venstre molar hos hannmus. Denne strategien utvidet området med bentap uten å ødelegge beinet rundt den andre molaren betydelig, noe som økte antallet forskjellige typer periodontalt vev.
«Vi isolerte tre hovedvevstyper og vurderte RNA-utbyttet mellom de to modellene. Resultatene viste at den trippelligerte modellen effektivt økte utbyttet, og oppnådde fire ganger mengden normalt periradikulært vev og støttet høyoppløselig analyse av forskjellige vevstyper», forklarer seniorforfatter Dr. Mikihito Hayashi.
Etter å ha bekreftet effektiviteten til den modifiserte modellen, satte forskerne seg fore å studere effekten av periodontitt på genuttrykk på tvers av ulike vevstyper over tid, med fokus på gener assosiert med betennelse og osteoklastdifferensiering.
Et av hovedfunnene deres var at uttrykket av Il1rl1-genet var betydelig høyere i det periradikulære vevet fem dager etter ligering. Dette genet koder for ST2-proteinet i reseptor- og lokkevevs-isoformer, som binder seg til et cytokin kalt IL-33, som er involvert i inflammatoriske og immunregulerende prosesser.
For å få ytterligere innsikt i rollen til dette genet, induserte teamet periodontitt hos genmodifiserte mus som manglet enten Il1rl1- eller Il33-genene. Disse musene viste akselerert inflammatorisk beinødeleggelse, noe som fremhever den beskyttende rollen til IL-33/ST2-signalveien. Videre analyse av celler som inneholder ST2-proteinet i reseptorformen mST2, viste at de fleste av dem stammet fra makrofager.
«Makrofager klassifiseres vanligvis i to hovedtyper, proinflammatoriske og antiinflammatoriske, avhengig av aktiveringen deres. Vi fant at mST2-uttrykkende celler var unike ved at de samtidig uttrykte noen markører for begge typer makrofager», kommenterte seniorforfatter Dr. Takanori Iwata. «Disse cellene var tilstede i det periradikulære vevet før betennelsen begynte, så vi kalte dem 'periodontalvevsresidente makrofager'.»
Samlet sett demonstrerer resultatene fra denne studien kraften til en modifisert dyremodell for å studere periodontitt i en mer detaljert skala, ned til biomolekylært nivå.
«Vi foreslår muligheten for en ny IL-33/ST2 molekylær bane som regulerer betennelse og beinødeleggelse ved periodontal sykdom, sammen med spesifikke makrofager i det periradikulære vevet som er dypt involvert i periodontal sykdom. Dette vil forhåpentligvis føre til utvikling av nye behandlingsstrategier og forebyggingsmetoder», konkluderer seniorforfatter Dr. Tomoki Nakashima.
