Nye publikasjoner
Forstå "hjertesorg" - studie finner sammenheng mellom stress og hjertesvikt
Sist anmeldt: 02.07.2025

Alt iLive-innhold blir gjennomgått med medisin eller faktisk kontrollert for å sikre så mye faktuell nøyaktighet som mulig.
Vi har strenge retningslinjer for innkjøp og kun kobling til anerkjente medieområder, akademiske forskningsinstitusjoner og, når det er mulig, medisinsk peer-evaluerte studier. Merk at tallene i parenteser ([1], [2], etc.) er klikkbare koblinger til disse studiene.
Hvis du føler at noe av innholdet vårt er unøyaktig, utdatert eller ellers tvilsomt, velg det og trykk Ctrl + Enter.

Stresset ved hjertesvikt huskes av kroppen og kan føre til tilbakefall og andre relaterte helseproblemer, viser en studie. Forskere har funnet ut at hjertesvikt etterlater et «stressminne» i form av endringer i DNA-et til hematopoietiske stamceller, som er involvert i å produsere blod og immunceller kalt makrofager.
Disse immuncellene spiller en viktig rolle i å beskytte hjertehelsen. Imidlertid ble en viktig signalvei (en kjede av molekyler som overfører signaler i en celle) kalt transformerende vekstfaktor beta (TGF-β) i hematopoietiske stamceller undertrykt under hjertesvikt, noe som påvirket makrofagproduksjonen negativt.
Forbedring av TGF-β-nivåene kan gi en ny behandling for tilbakevendende hjertesvikt, og det å oppdage akkumulering av stressminne kan gi en tidlig advarsel før det oppstår.
Sunn livsstil og forbedret velvære er en del av FNs globale bærekraftsmål. Positivt nok viser en fersk studie at forventet levealder på verdensbasis vil øke med rundt 4,5 år innen 2050. Dette skyldes i stor grad folkehelsearbeidet for å forebygge sykdom og forbedre overlevelse fra tilstander som hjerte- og karsykdommer. Hjertesykdom er imidlertid fortsatt den ledende dødsårsaken på verdensbasis, med anslagsvis 26 millioner mennesker som lider av hjertesvikt.
Når hjertesvikt først oppstår, har den en tendens til å komme tilbake, ledsaget av andre helseproblemer som nyre- og muskelsykdom. Forskere i Japan ønsket å forstå hva som forårsaker disse tilbakefallene og forverringen av andre organer, og om det kan forebygges.
Studien er publisert i tidsskriftet Science Immunology.
«Basert på våre tidligere studier, antok vi at tilbakefall kan være forårsaket av stress som oppleves under hjertesvikt, som akkumuleres i kroppen, spesielt i hematopoietiske stamceller», forklarte prosjektprofessor Katsuito Fujio ved University of Tokyo Graduate School of Medicine. Hematopoietiske stamceller finnes i beinmargen og er kilden til blodceller og immunceller kalt makrofager, som bidrar til å beskytte hjertehelsen.
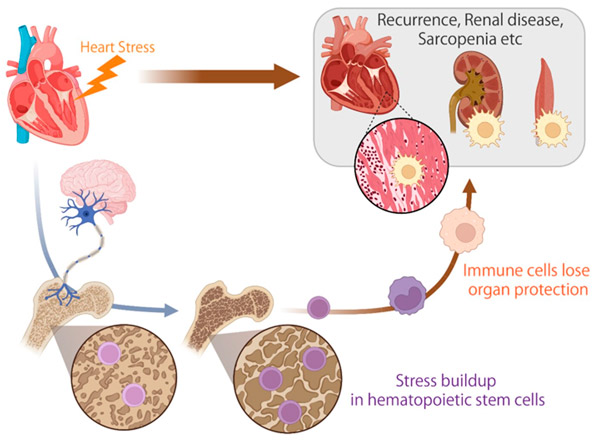
Denne illustrasjonen viser at stresssignaler overføres til hjernen under hjertesvikt, som deretter sender dem videre via nerver til hematopoietiske stamceller i benmargen, der de akkumuleres som stressminne. Disse stressakkumulerte stamcellene produserer immunceller med reduserte beskyttelsesevner for organer som hjerte, nyrer og muskler. Kilde: Science Immunology (2024). DOI: 10.1126/sciimmunol.ade3814
Hos mus med hjertesvikt fant forskerne tegn på stresspreging på epigenomet, som betyr at det hadde skjedd kjemiske endringer i musenes DNA. En viktig signalvei kalt transformerende vekstfaktor beta, som er involvert i reguleringen av mange cellulære prosesser, ble undertrykt i de hematopoietiske stamcellene hos mus med hjertesvikt, noe som førte til produksjon av dysfunksjonelle immunceller.
Disse endringene vedvarte over tid, så da teamet transplanterte benmarg fra mus med hjertesvikt til friske mus, fant de ut at stamcellene fortsatte å produsere dysfunksjonelle immunceller. Disse musene utviklet deretter hjertesvikt og ble utsatt for organskade.
«Vi kalte dette fenomenet et stressminne fordi stresset ved hjertesvikt huskes lenge og fortsetter å påvirke hele kroppen. Selv om ulike andre typer stress også kan etterlate dette stressminnet, tror vi at stresset forårsaket av hjertesvikt er spesielt betydelig», sa Fujio.
Den gode nyheten er at det å identifisere og forstå disse endringene i TGF-β-signalveien åpner for nye muligheter for potensielle fremtidige behandlinger.
«Helt nye terapier kan vurderes for å forhindre akkumulering av dette stressminnet under sykehusinnleggelse ved hjertesvikt», sa Fujio. «Hos dyr med hjertesvikt har tilsetning av ytterligere aktiv TGF-β vist potensial som et behandlingsalternativ. Korrigering av epigenomet til hematopoietiske stamceller kan også være en måte å eliminere stressminnet på.»
Nå som dette er identifisert, håper teamet å utvikle et system som kan oppdage og forhindre opphopning av stressminne hos mennesker, med det langsiktige målet å ikke bare forhindre at hjertesvikt kommer tilbake, men også identifisere tilstanden før den utvikler seg fullt ut.
