Nye publikasjoner
Forskere modifiserte E. coli med deler av HIV-viruset for å utvikle en vellykket vaksine
Sist anmeldt: 02.07.2025

Alt iLive-innhold blir gjennomgått med medisin eller faktisk kontrollert for å sikre så mye faktuell nøyaktighet som mulig.
Vi har strenge retningslinjer for innkjøp og kun kobling til anerkjente medieområder, akademiske forskningsinstitusjoner og, når det er mulig, medisinsk peer-evaluerte studier. Merk at tallene i parenteser ([1], [2], etc.) er klikkbare koblinger til disse studiene.
Hvis du føler at noe av innholdet vårt er unøyaktig, utdatert eller ellers tvilsomt, velg det og trykk Ctrl + Enter.
Nikolay Shcherbak, førsteamanuensis i biologi ved Örebro universitet, har nettopp returnert til Sverige etter å ha deltatt på en konferanse i Sør-Afrika, hvor han presenterte forskning som øker sjansene for å utvikle en HIV-vaksine. Sammen med andre forskere modifiserte han den probiotiske bakterien E. coli genetisk for å inkludere deler av HIV-viruset.
Artikkelen er publisert i tidsskriftet Microbial Cell Factories.
«Ved hjelp av banebrytende teknologi setter vi inn DNA-sekvenser på et spesifikt sted i bakteriene. Vi bruker en del av HIV-viruset som ikke er smittsomt, men som likevel får kroppen til å produsere nøytraliserende antistoffer», sier Shcherbak.
E. coli-bakterier lever i tarmene til mennesker og andre dyr, og noen varianter av dem forårsaker ulike typer infeksjoner. Det finnes imidlertid også gunstige varianter av disse bakteriene som kan bidra til å forbedre tarmfloraen. En slik bakterie, den probiotiske E. coli Nissle-stammen, ble brukt av Örebro-forskerne i studien sin.
«Bakteriene vi bruker selges som kosttilskudd i Tyskland, men så vidt jeg vet er de ikke tilgjengelige i Sverige. Disse kosttilskuddene anbefales for personer med irritabel tarm-syndrom (IBS) eller andre magelidelser.»
HIV er et virus som kan forårsake den dødelige immunsviktsykdommen AIDS, som det ikke finnes noen kur for. Det finnes imidlertid medisiner for å behandle HIV som gjør at smittede mennesker kan leve uten symptomer eller risiko for å overføre sykdommen.
«En HIV-smittet person må ta antiretrovirale legemidler resten av livet, og prisen kan være uoverkommelig for alle. Forskere har utviklet en vaksine i mange år, men dessverre er det ikke en prioritet for legemiddelselskaper», sier Shcherbak.
Hvis bakteriene som utvikles ved Örebro universitet resulterer i et godkjent farmasøytisk produkt, kan det tas i tablettform. Vaksiner i tablettform har betydelige fordeler fremfor vaksiner som må injiseres. Tabletter er enklere og mer praktiske å bruke, og de trenger ikke å oppbevares ved lave temperaturer, slik som noen COVID-19-vaksiner gjør.
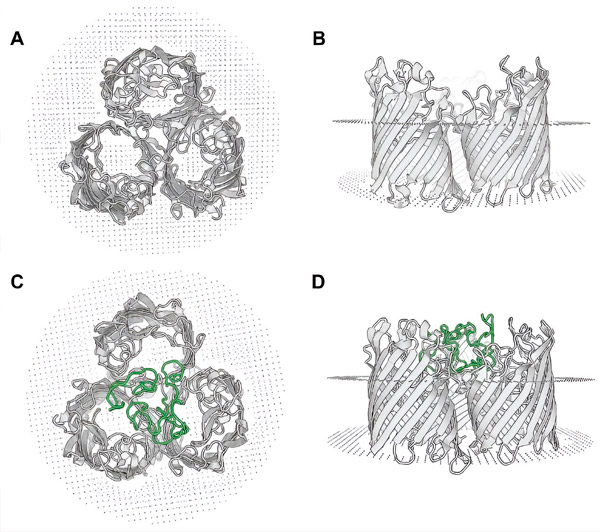
Homolog modellering av det rekombinante OmpF-MPER-proteinet. Toppvisning (A) og sidevisning (B) av OmpF-proteintrimeren fra E. coli K-12-stammen (basert på 6wtz.pdb). Toppvisning (C) og sidevisning (D) av det predikerte OmpF-MPER-proteinet fra EcN-MPER, homologimodellering utført på 6wtz.pdb-strukturen ved hjelp av SWISS-MODEL-verktøyet. Plasseringen av MPER-sekvensen er angitt med grønt. Kilde: Microbial Cell Factories (2024). DOI: 10.1186/s12934-024-02347-8
I mange tidligere forsøk på å bruke bakterier til å produsere vaksiner har forskere brukt antibiotikaresistensgener for å opprettholde genetiske modifikasjoner i bakterier. Denne metoden kan imidlertid føre til negative konsekvenser som antibiotikaresistens, som er et voksende globalt folkehelseproblem. Ved hjelp av CRISPR/Cas9-teknologi har forskere fra Örebro laget stabil genetisk modifikasjon i probiotiske bakterier uten behov for antibiotikaresistensgener.
Shcherbak ser ingen risikoer ved bruk av genmodifiserte bakterier. Det er imidlertid behov for mer forskning, inkludert dyreforsøk, før teknologien kan testes på mennesker og en vaksine kan se dagens lys.
«Det tar minst et par år å forberede seg og få etiske godkjenninger. Under normale forhold tar legemiddelutvikling omtrent ti år», sier Shcherbak.
