Nye publikasjoner
Forskere har skapt en "kameleon"-forbindelse for å behandle medisinresistent hjernekreft
Sist anmeldt: 02.07.2025

Alt iLive-innhold blir gjennomgått med medisin eller faktisk kontrollert for å sikre så mye faktuell nøyaktighet som mulig.
Vi har strenge retningslinjer for innkjøp og kun kobling til anerkjente medieområder, akademiske forskningsinstitusjoner og, når det er mulig, medisinsk peer-evaluerte studier. Merk at tallene i parenteser ([1], [2], etc.) er klikkbare koblinger til disse studiene.
Hvis du føler at noe av innholdet vårt er unøyaktig, utdatert eller ellers tvilsomt, velg det og trykk Ctrl + Enter.
En ny studie utført av forskere ved Yale University beskriver hvordan en ny kjemisk forbindelse angriper medikamentresistente hjernesvulster uten å skade sunt omkringliggende vev.
Studien, publisert i Journal of the American Chemical Society, er et viktig skritt i utviklingen av såkalte «kameleonforbindelser» som kan brukes til å bekjempe en rekke farlige kreftformer.
Gliomer utvikler seg hos omtrent 6,6 per 100 000 personer hvert år og hos 2,94 per 100 000 personer innen 14-årsalderen. Bortsett fra metastaser fra andre kreftformer som når sentralnervesystemet, står gliomer for 26 % av alle hjernesvulster (primære hjernesvulster) og 81 % av alle ondartede hjernesvulster.
I flere tiår har pasienter med glioblastom blitt behandlet med et legemiddel som heter temozolomid. De fleste pasienter utvikler imidlertid resistens mot temozolomid innen et år. Femårsoverlevelsesraten for pasienter med glioblastom er mindre enn 5 %.
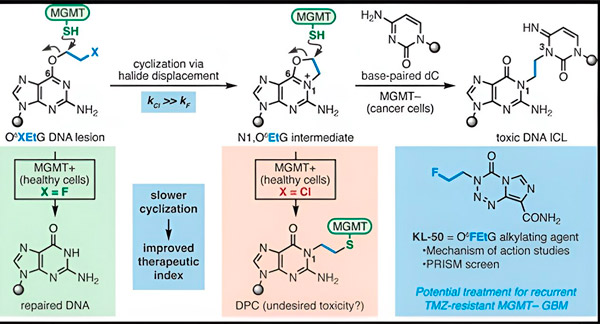
I 2022 utviklet Yale-kjemikeren Seth Herzon og stråleonkolog Dr. Ranjit Bindra en ny strategi for å behandle glioblastomer mer effektivt. De skapte en klasse antikreftmolekyler kalt kameleonforbindelser som utnytter en defekt i et DNA-reparasjonsprotein kjent som O6-metylguanin-DNA-metyltransferase (MGMT).
Mange kreftceller, inkludert glioblastomer, mangler MGMT-proteinet. Nye kameleonforbindelser er utviklet for å skade DNA i tumorceller som mangler MGMT.
Kameleonforbindelser initierer DNA-skade ved å avsette primære lesjoner på DNA som over tid utvikler seg til svært giftige sekundære lesjoner kjent som interstrand-tverrbindinger. MGMT beskytter DNA-et i sunt vev ved å reparere primære lesjoner før de kan utvikle seg til dødelige interstrand-tverrbindinger.
For den nye studien fokuserte medforfatterne Herzon og Bindra på sin ledende kameleon, KL-50.
«Vi brukte en kombinasjon av syntetisk kjemi og molekylærbiologiske studier for å belyse det molekylære grunnlaget for våre tidligere observasjoner, samt den kjemiske kinetikken som gir den unike selektiviteten til disse forbindelsene», sa Herzon, Milton Harris-professor i kjemi ved Yale. «Vi viser at KL-50 er unik ved at den kun danner DNA-tverrbindinger i svulster med defekt DNA-reparasjon. Den skåner sunt vev.»
Kilde: Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.3c06483
Det er en betydelig forskjell, påpeker forskerne. En rekke andre kreftbekjempende forbindelser er utviklet for å utløse tverrbindinger mellom strengene, men de er ikke selektive for tumorceller, noe som begrenser nytten av dem.
Hemmeligheten bak KL-50s suksess er timingen, bemerket forskerne. KL-50 danner tverrbindinger mellom strengene saktere enn andre tverrbindingsmidler. Denne forsinkelsen gir friske celler nok tid til å bruke MGMT for å forhindre tverrbindinger i å dannes.
«Denne unike profilen antyder potensialet for behandling av medikamentresistent glioblastom, et område med et stort udekket behov i klinikken», sa Bindra, Harvey og Kate Cushing-professor i terapeutisk radiologi ved Yale School of Medicine. Bindra er også vitenskapelig leder for Chenevert Family Brain Tumor Center ved Smilo Hospital.
Herzon og Bindra sa at studien deres fremhever viktigheten av å vurdere hastighetene på kjemisk DNA-modifisering og biokjemisk DNA-reparasjon. De tror de kan bruke denne strategien til å utvikle behandlinger for andre kreftformer som inneholder spesifikke tumorassosierte DNA-reparasjonsdefekter.
