Nye publikasjoner
Ny studie avslører mitokondrielle proteiners nøkkelrolle i regenerering av hjertet
Sist anmeldt: 02.07.2025

Alt iLive-innhold blir gjennomgått med medisin eller faktisk kontrollert for å sikre så mye faktuell nøyaktighet som mulig.
Vi har strenge retningslinjer for innkjøp og kun kobling til anerkjente medieområder, akademiske forskningsinstitusjoner og, når det er mulig, medisinsk peer-evaluerte studier. Merk at tallene i parenteser ([1], [2], etc.) er klikkbare koblinger til disse studiene.
Hvis du føler at noe av innholdet vårt er unøyaktig, utdatert eller ellers tvilsomt, velg det og trykk Ctrl + Enter.
Mitokondrier spiller en kritisk rolle i å gi energien som trengs for riktig cellefunksjon. I mitokondrier produseres energi av respirasjonskjeden, som består av fem komplekser, betegnet CI-CV. Disse kompleksene kan settes sammen til superkomplekser, men lite er kjent om rollen til denne prosessen og dens kontroll.
Den nye studien undersøker mekanismene bak superkompleks-samling og avslører en betydelig innvirkning av mitokondrielle samlingsfaktorer på regenerering av hjertevev. Studien ble ledet i fellesskap av Dr. José Antonio Enríquez fra National Center for Cardiovascular Research (CNIC) og Dr. Nadia Mercader fra Universitetet i Bern i Sveits, som er gjesteforsker ved CNIC.
En studie publisert i tidsskriftet Developmental Cell viser at proteinfamiliemedlemmet Cox7a spiller en grunnleggende rolle i sammensetningen av CIV-dimerer, og at denne sammensetningen er kritisk for at mitokondriene skal fungere ordentlig og dermed for cellulær energiproduksjon.
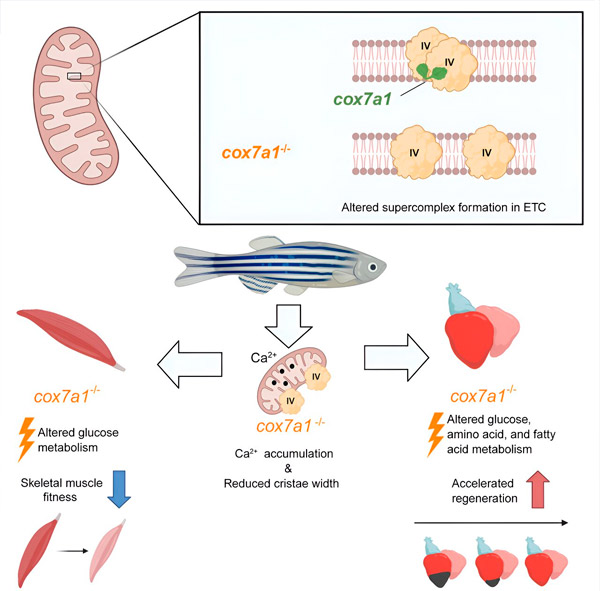
Cox7a-proteinfamilien omfatter tre medlemmer: Cox7a1, Cox7a2 og Cox7a2l (også kalt SCAF1). Tidligere studier av begge gruppene har vist at når CIV inneholder SCAF1, assosieres det sterkt med CIII, og danner et respiratorisk superkompleks kjent som respirasomet. I disse tidligere studiene antok forfatterne at inkludering av Cox7a2 ville resultere i CIV som ikke er i stand til å assosiere, mens CIV-molekyler som inneholder Cox7a1 ville assosiere for å danne CIV-homodimerer. Den nye studien demonstrerer eksperimentelt en rolle for Cox7a1 i dannelsen av disse CIV-homodimerene.
Utviklingscelle (2024). DOI: 10.1016/j.devcel.2024.04.012
Forskerne jobbet med en sebrafiskmodell og fant at fraværet av Cox7a1 forhindret dannelsen av CIV-dimerer, og tapet av disse dimerene påvirket vekten og svømmeevnen til den berørte fisken.
«Cox7a1 uttrykkes primært i tverrstripete muskelceller, og det var skjelettmuskelvev som led mest av mangelen på Cox7a1-funksjon. Den andre hovedtypen av tverrstripet muskel er hjertemuskel, eller myokard,» forklarte Dr. Enriquez.
Imidlertid, mens tap av Cox7a1 i skjelettmuskulatur var skadelig, forbedret fraværet av Cox7a1 i hjertemuskulaturen den kardiale regenerative responsen på skade.
«Dette resultatet viser at disse proteinene spiller en nøkkelrolle i å aktivere hjertets evne til å reparere seg selv etter skade», forklarte Carolina Garcia-Pojatos, førsteforfatter av studien.
For å utforske funksjonen til Cox7a1 ytterligere, utførte CNIC-forskerne Enrique Calvo og Jesús Vásquez en proteomisk studie av skjelettmuskulatur og myokard hos sebrafisk som manglet Cox7a1. Denne analysen ble supplert med en metabolomisk studie utført av kolleger ved Universitetet i Bern. Denne kombinerte analysen avdekket signifikante forskjeller fra umodifisert fisk med intakt Cox7a1-ekspresjon.
«Disse resultatene tyder på at molekyler involvert i sammensetningen av mitokondrielle superkomplekser kan ha betydelige effekter på metabolsk kontroll, og muligens åpne veien for nye behandlinger for hjertesykdom og andre metabolske tilstander», sa Dr. Mercader.
Ifølge forskerteamet representerer denne oppdagelsen «et betydelig skritt fremover i forståelsen av de cellulære mekanismene som er involvert i hjerteregenerering, og kan peke veien for utviklingen av terapier som tar sikte på å stimulere hjerteregenerering».
Forfatterne konkluderer med at mitokondrielle samlingsfaktorer kan påvirke metabolsk kontroll betydelig.
