Nye publikasjoner
Viktig nevron som kontrollerer bevegelse hos ormer oppdaget, viktig for behandling av mennesker
Sist anmeldt: 02.07.2025

Alt iLive-innhold blir gjennomgått med medisin eller faktisk kontrollert for å sikre så mye faktuell nøyaktighet som mulig.
Vi har strenge retningslinjer for innkjøp og kun kobling til anerkjente medieområder, akademiske forskningsinstitusjoner og, når det er mulig, medisinsk peer-evaluerte studier. Merk at tallene i parenteser ([1], [2], etc.) er klikkbare koblinger til disse studiene.
Hvis du føler at noe av innholdet vårt er unøyaktig, utdatert eller ellers tvilsomt, velg det og trykk Ctrl + Enter.

Forskere fra Sinai Health og University of Toronto har oppdaget en mekanisme i nervesystemet til den lille rundormen C. elegans som kan ha betydelige implikasjoner for behandling av menneskelige sykdommer og utviklingen av robotikk.
Studien, ledet av Mei Zhen og kolleger ved Lunenfeld-Tanenbaum Research Institute, er publisert i tidsskriftet Science Advances og avslører nøkkelrollen til et spesifikt nevron kalt AVA i å kontrollere ormens evne til å veksle mellom bevegelse fremover og bakover.
Det er viktig for ormer å krype mot matkilder og raskt trekke seg tilbake fra fare. Denne oppførselen, der de to handlingene utelukker hverandre, er typisk for mange dyr, inkludert mennesker, som ikke kan sitte og løpe samtidig.
Forskere har lenge trodd at bevegelseskontroll hos ormer oppnås gjennom det enkle samspillet mellom to nevroner: AVA og AVB. Førstnevnte ble antatt å fremme bevegelse bakover, sistnevnte bevegelse fremover, hvor hver hemmer den andre for å kontrollere bevegelsesretningen.
Nye data fra Zhens team utfordrer imidlertid dette synet, og avslører en mer kompleks interaksjon der AVA-nevronet spiller en dobbel rolle. Ikke bare stopper det umiddelbart bevegelse fremover ved å undertrykke AVB, men det opprettholder også langsiktig stimulering av AVB for å sikre en jevn overgang tilbake til bevegelse fremover.
Denne oppdagelsen fremhever AVA-nevronets evne til å finkontrollere bevegelse gjennom forskjellige mekanismer, avhengig av forskjellige signaler og på forskjellige tidsskalaer.
«Fra et ingeniørperspektiv er dette en svært økonomisk design», sier Zheng, professor i molekylærgenetikk ved University of Torontos Temerty School of Medicine. «Sterk, vedvarende hemming av tilbakekoblingssløyfen lar dyret reagere på ugunstige forhold og unnslippe. Samtidig fortsetter kontrollnevronet å pumpe en konstant gass inn i fremoversløyfen for å bevege seg til trygge steder.»
Jun Meng, en tidligere doktorgradsstudent i Zhengs laboratorium som ledet studien, sa at det å forstå hvordan dyr går over mellom slike motstridende motoriske tilstander er nøkkelen til å forstå hvordan dyr beveger seg, samt for forskning på nevrologiske lidelser.
Oppdagelsen av AVA-nevronets dominerende rolle gir ny innsikt i nevrale kretser som forskere har studert siden moderne genetikk kom for mer enn et halvt århundre siden. Zhengs laboratorium brukte vellykket banebrytende teknologi for å modulere aktiviteten til individuelle nevroner presist og registrere data fra levende ormer i bevegelse.
Zhen, som også er professor i celle- og systembiologi ved University of Torontos fakultet for kunst og vitenskap, understreker viktigheten av tverrfaglig samarbeid i denne studien. Meng utførte de viktigste eksperimentene, og elektriske opptak fra nevroner ble utført av Bin Yu, en doktorgradsstudent i Shangbang Gaos laboratorium ved Huazhong University of Science and Technology i Kina.
Tosif Ahmed, en tidligere postdoktor i Zhengs laboratorium og nå teoriforsker ved HHMIs Janelia Research Campus i USA, ledet den matematiske modelleringen som var viktig for å teste hypoteser og få ny innsikt.
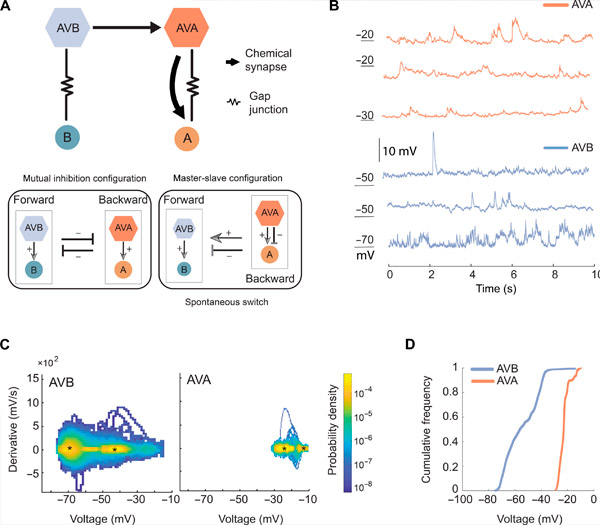
AVA og AVB har forskjellige membranpotensialområder og dynamikk. Kilde: Science Advances (2024). DOI: 10.1126/sciadv.adk0002
Studiens funn gir en forenklet modell for å studere hvordan nevroner kan håndtere flere roller i bevegelseskontroll – et konsept som også kan brukes på menneskelige nevrologiske tilstander.
For eksempel avhenger AVAs doble rolle av dens elektriske potensial, som reguleres av ionekanaler på overflaten. Zheng undersøker allerede hvordan lignende mekanismer kan være involvert i en sjelden tilstand kjent som CLIFAHDD-syndrom, forårsaket av mutasjoner i lignende ionekanaler. De nye funnene kan også informere utformingen av mer adaptive og effektive robotsystemer som er i stand til å utføre komplekse bevegelser.
«Fra moderne vitenskaps opprinnelse til banebrytende forskning i dag har modellorganismer som C. elegans spilt en viktig rolle i å avdekke kompleksiteten i våre biologiske systemer», sa Anne-Claude Gingras, direktør for Lunenfeld-Tanenbaum Research Institute og visepresident for forskning ved Sinai Health. «Denne studien er et godt eksempel på hvordan vi kan lære av enkle dyr og anvende den kunnskapen til å fremme medisin og teknologi.»
