Nye publikasjoner
Protein ansvarlig for genetisk inflammatorisk sykdom identifisert
Sist anmeldt: 02.07.2025

Alt iLive-innhold blir gjennomgått med medisin eller faktisk kontrollert for å sikre så mye faktuell nøyaktighet som mulig.
Vi har strenge retningslinjer for innkjøp og kun kobling til anerkjente medieområder, akademiske forskningsinstitusjoner og, når det er mulig, medisinsk peer-evaluerte studier. Merk at tallene i parenteser ([1], [2], etc.) er klikkbare koblinger til disse studiene.
Hvis du føler at noe av innholdet vårt er unøyaktig, utdatert eller ellers tvilsomt, velg det og trykk Ctrl + Enter.
Et forskerteam ledet av Dr. Hirotsugu Oda fra CECAD Cluster of Excellence for Aging Research ved Universitetet i Köln har oppdaget hvilken rolle et bestemt proteinkompleks spiller i noen former for immunforstyrrelser. Dette resultatet kan føre til utvikling av nye terapeutiske tilnærminger som tar sikte på å redusere autoinflasjon og «gjenopprette» immunforsvaret til pasienter som lider av en genetisk dysfunksjon av dette proteinkomplekset.
Studien, «Ballelisk human SHARPIN-tap av funksjon induserer autoinflammasjon og immunsvikt», ble publisert i tidsskriftet Nature Immunology.
Det lineære ubiquitin-assemblerende komplekset (LUBAC), som består av proteinene HOIP, HOIL-1 og SHARPIN, har lenge vært anerkjent for sin kritiske rolle i å opprettholde immunhomeostase. Tidligere studier på mus har vist de alvorlige konsekvensene av SHARPIN-tap, som fører til alvorlig dermatitt på grunn av overdreven hudcelledød. Imidlertid har de spesifikke helsekonsekvensene av SHARPIN-mangel hos mennesker forblitt uklare.
Forskerteamet rapporterte for første gang to personer med SHARPIN-mangel som viser symptomer på autoinflasjon og immunsvikt, men uventet nok ikke viser dermatologiske problemer slik de gjorde hos mus.
Ved nærmere undersøkelse ble det funnet at disse individene hadde en svekket kanonisk NF-κB-respons, en viktig immunresponsvei. De hadde også økt mottakelighet for celledød indusert av medlemmer av tumornekrosefaktor (TNF)-superfamilien. Behandling av én SHARPIN-mangelfull pasient med anti-TNF-behandling, som spesifikt hemmer TNF-indusert celledød, resulterte i fullstendig opphør av autoinflasjon på cellenivå og i klinisk presentasjon.
Studien viser at overdreven og ukontrollert celledød spiller en kritisk rolle i genetiske inflammatoriske sykdommer hos mennesker. Odas team la til SHARPIN-mangel som et nytt medlem av en gruppe genetiske inflammatoriske sykdommer hos mennesker de foreslår å kalle «medfødte celledødsfeil».
Beskyttelse mot immunforstyrrelser Studien ble startet i laboratoriet til Dr. Dan Kastner ved National Institutes of Health (NIH) i USA. Forskerne der fikk muligheten til å observere en pasient med uforklarlige episoder med feber, leddgikt, kolitt og immunsvikt som debuterte i barndommen.
Etter å ha innhentet informert samtykke, utførte de eksomsekvensering på pasienten og hans familiemedlemmer, og fant at pasienten hadde en ødeleggende genetisk variant i SHARPIN-genet som førte til uoppdagbare nivåer av SHARPIN-proteinet. De fant også at pasientens celler viste en økt tilbøyelighet til å dø i både dyrkede celler og i biopsier fra pasienten.
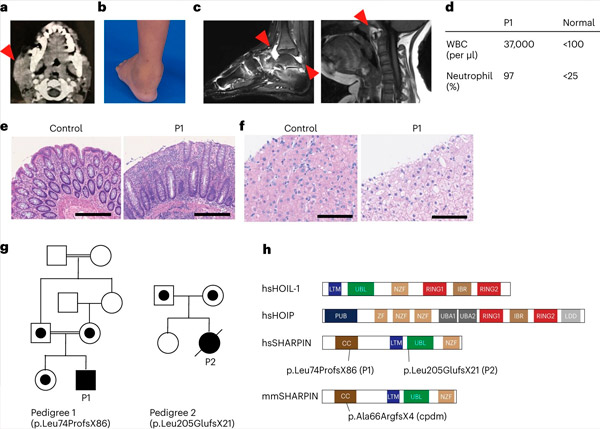
SHARPIN-mangel hos mennesker forårsaker autoinflammasjon og leverglykogenose. Kilde: Nature Immunology (2024). DOI: 10.1038/s41590-024-01817-w
Teamet fant også at utviklingen av lymfoide germinale sentre – spesialiserte mikrostrukturer i adenoidene som er kritiske for modningen av immunsystemets B-celler og dermed for antistoffproduksjon – ble betydelig redusert på grunn av økt B-celledød. Disse funnene forklarer pasientenes immunsvikt og fremhever den viktige rollen LUBAC spiller i å opprettholde immunhomeostase hos mennesker.
«Studien vår fremhever den kritiske betydningen av LUBAC for å beskytte mot immunforstyrrelser. Ved å belyse de molekylære mekanismene som ligger til grunn for LUBAC-mangel, baner vi vei for nye terapeutiske strategier som tar sikte på å gjenopprette immunhomeostase», sa Oda, hovedforfatter av studien.
Han la til: «En av pasientene med SHARPIN-mangel hadde vært rullestolbundet i årevis før vi så ham første gang. Anklene hans var betente, og det var for smertefullt å gå. Den genetiske diagnosen lot oss målrette den riktige molekylære banen som ligger til grunn for tilstandene hans.»
Siden pasienten begynte å motta anti-TNF-behandling, har han vært symptomfri i nesten syv år. «Som kliniker og forsker er jeg glad for å ha muligheten til å påvirke én pasients liv positivt gjennom forskningen vår», konkluderte Oda.
